
Downloadable Periodic Table Oxidation States
Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds.. 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups. Periodic Table of Elements. Properties Electrons Isotopes Compounds. or buying a poster or wallet card, order number. 1H Hydrogen 1..
What is Oxidation State?
This black and white printable periodic table chart is an updated version of the Printable Periodic Table - Element Charges. Each element cell contains the atomic number, symbol, name, atomic mass and most common valence charge or oxidation state of each element. The table is available for download in PDF format for offline printing. For best.

Pin by Natalie Paes on chem Chemistry education, Chemical science
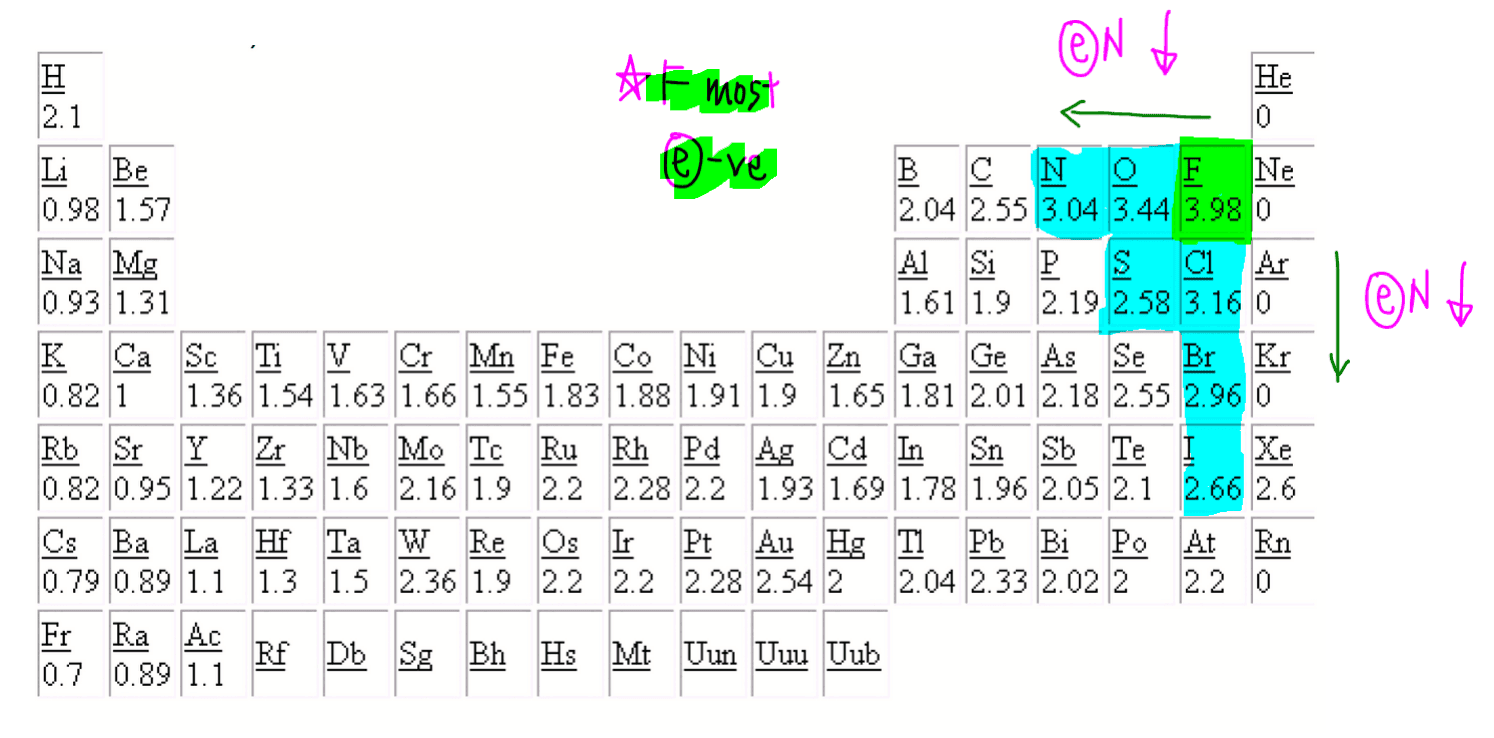
However, most metals are capable of multiple oxidation states. For example, iron common has an oxidation number of +2 or +3. Halogens, on the other hand, have an oxidation state of -1. This periodic table contains the oxidation numbers of the elements as well as element numbers, symbols, names, and atomic weights.

Oxidation Numbers Periodic Table Elements
Color of the element symbol shows state of matter: black=solid: white=liquid: red=gas: grey=unknown
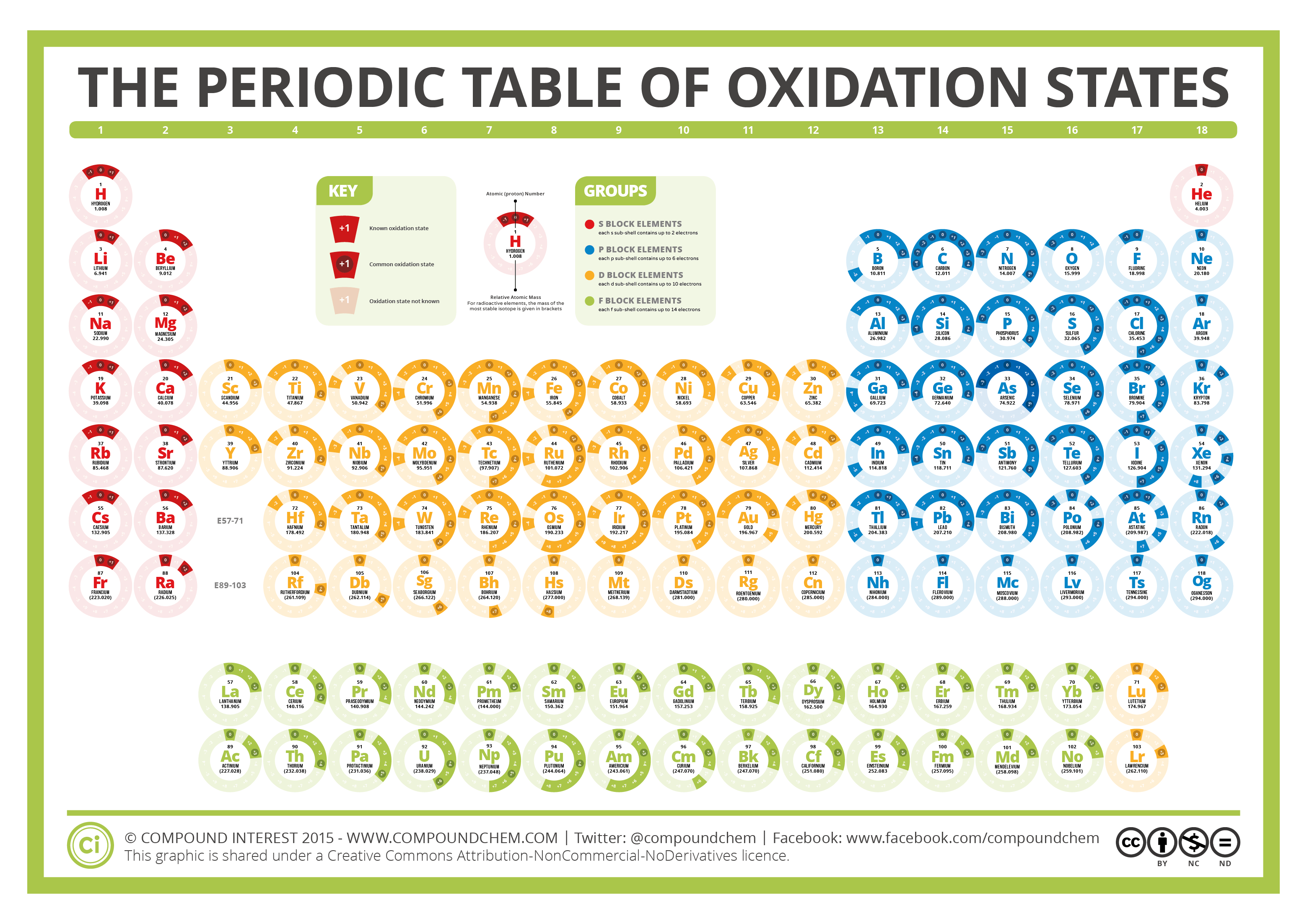
The Periodic Table of Oxidation States Compound Interest
If oxygen is present, assume oxygen is -2. We created a video to explain how this is done. Rule 10 oxidation number examples: For example, in the nitrate ion NO 3-, the charge of the ion is -1. Then, using rule 2, we know the charge of the oxygens is 3 x-2 = -6. So nitrogen must have a charge of +5 to make the charge of the ion -1.

Oxidation Number (State) Definition, Rules, How to Find, and Examples
This number is defined as the formal charge on the atom if all bonds were assumed to be fully ionic. Knowing the oxidation state is very useful for example when balancing reduction-oxidation (redox) reactions. Figure 1: Oxidation states in the Periodic Table. Referred from: Article. Oxidation numbers for atoms, ions and compounds.
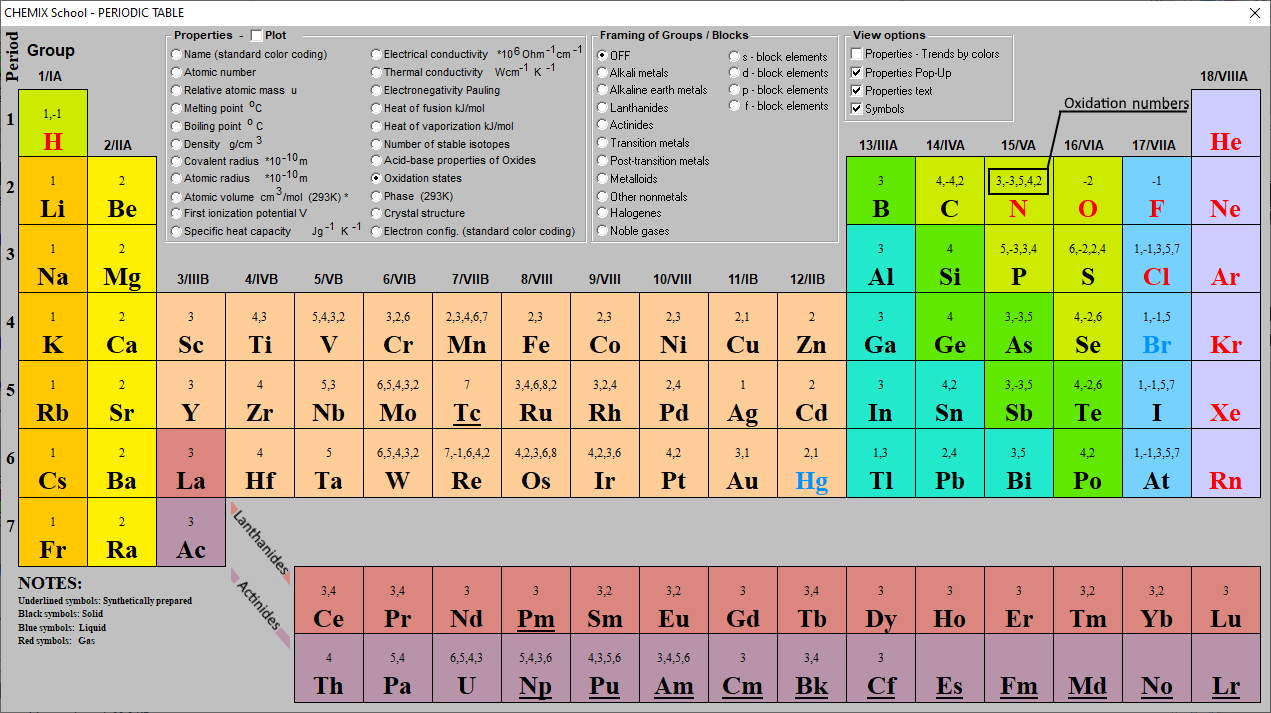
Oxidation Numbers Periodic Table Elements
This printable periodic table contains the number, symbol, name, atomic mass and oxidation states of each element. The most common oxidation states are in bold text and predicted or unconfirmed states are in italics. This table is available for download as a PDF file and printed for offline use. For best printing, choose Landscape and 'Fit.
/PeriodicTableOxidation-BW-56a12da83df78cf772682bfe.png)
Periodic Table of the Elements Oxidation Numbers
Hydroxide is an anion (negatively charged ion) made up of one oxygen atom to one hydrogen atom. Since oxygen is highly electronegative it is a common oxidizing agent. The oxidation number for O is 2- in most compounds. In this video, 2- is written above O. Another way to think about it is 1- being written above O2.
/PeriodicTableOxidation-BW-56a12da83df78cf772682bfe.png)
Periodic Table With Groups Numbers Periodic Table Timeline
To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'Calculate' (for example: Ca2+, HF2^-, Fe4 [Fe (CN)6]3, NH4NO3, so42-, ch3cooh, cuso4*5h2o ). The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds. The oxidation number is synonymous with.

How to Assign Oxidation Numbers
This color periodic table contains the number, symbol, name, atomic mass and oxidation states of each element. The most common oxidation states are in bold text and predicted or unconfirmed states are in italics. This table is available for download as a PDF file and printed for offline use. For best printing, choose Landscape and 'Fit' for.

Downloadable Periodic Table Oxidation States
Oxidation number of an atom when an element has combined with the same element. Table: oxidation numbers of according to the atomic number, first 20 elements. Oxidation states of s block. Hydrogen. Alkali Metals - Group 1. Alkali Earth Metals - Group 2. Oxidation states of p block elements. Group 3. Group 4.
:max_bytes(150000):strip_icc()/PeriodicTableOxidation-BW-56a12da83df78cf772682bfe.png)
Periodic Table of the Elements Oxidation Numbers
Chemical Element Data in PubChem. PubChem is providing this periodic table page in order to help navigate abundant chemical element data available in PubChem. When exploring the table or list views on this page, please note the links to dedicated pages for each element. These individual element summary pages contain a lot of additional.
Aluminum Aluminum Oxidation Number
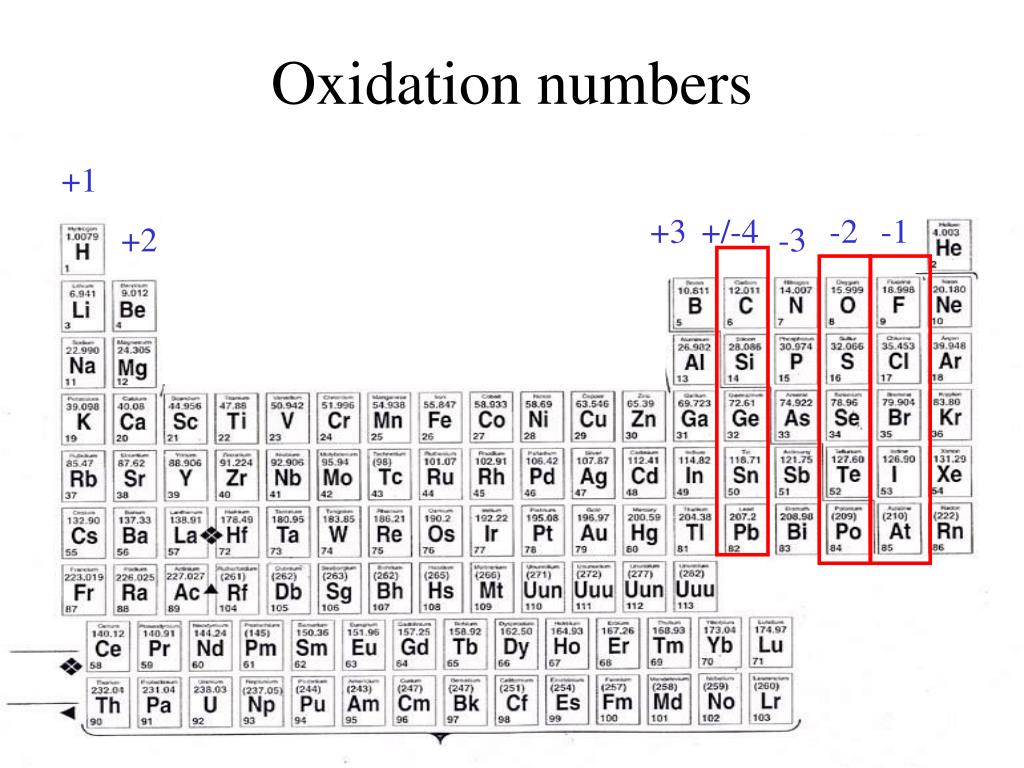
Po. +2. 85. At. -1. 86. Rn. The more common oxidation numbers are in color. The oxidation number +3 is common to all lanthanides and actinides in their compounds.

Free Printable Periodic Tables (PDF and PNG) Science Notes and Projects
These have an oxidation state of +1, the same as the charge on the ion. Similarly, iron (Fe) can lost two electrons to form the Fe 2+ ion, or lose three electrons to form the Fe 3+ ion. These have oxidation numbers of +2 & +3 respectively. With a chlorine ion (a chlorine atom that has gained one electron, Cl - ), the oxidation number would be.
PPT Elements and Periodic Table PowerPoint Presentation, free
The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. Alkali metals - elements in the first column of the periodic table - will always have an oxidation number of +1; Alkali metals (column 2) are almost always +2
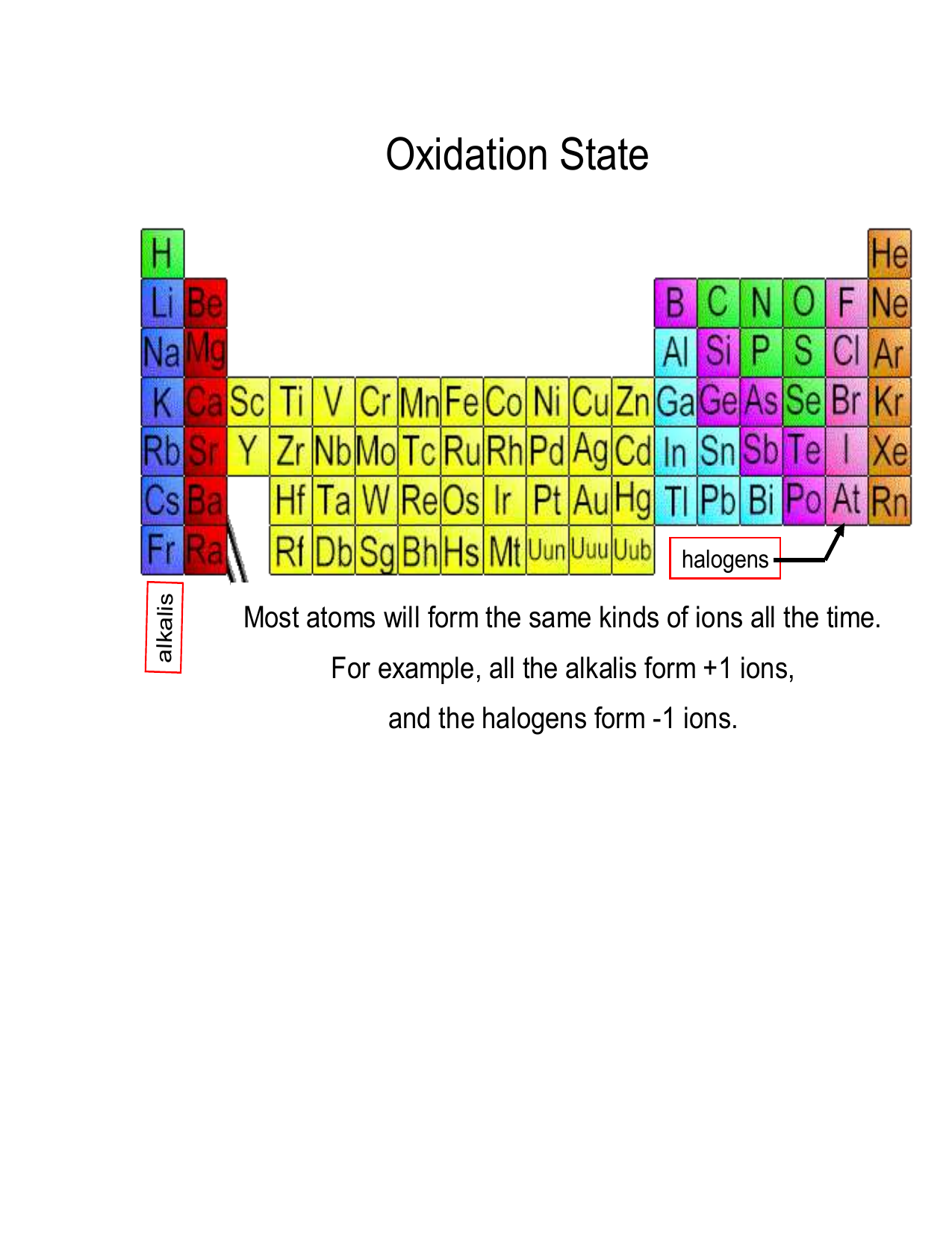
Oxidation Numbers
Oxidation numbers; Monoatomic ions oxidation numbers; Polyatomic ions oxidation numbers; Periodic table with oxidation numbers; Steps to writing chemical formulas; Molecular and Empirical Formulas; Molecular mass and formula mass; Types of chemical compounds; Binary compounds; Ternary compounds